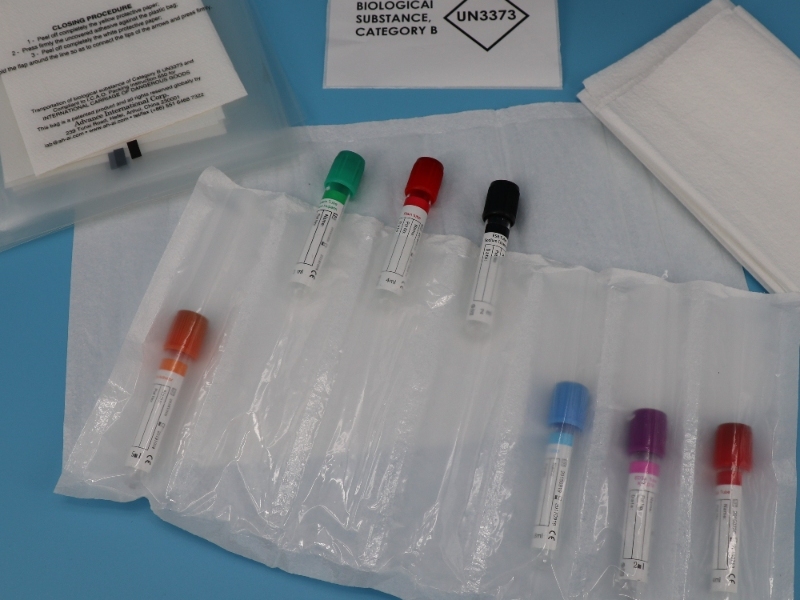
Enhanced Specimen Transport Safety: New Generation of Bags Addresses Critical Lab and Field Needs
Release time: 2025-08-19
Medical laboratories and diagnostic services worldwide are adopting advanced specimen carrier bag systems to address growing safety and compliance demands in biological sample transport. A key innovation driving this shift is the integration of 95kPa bags – rigorously tested containers capable of withstanding significant pressure differentials encountered during air transport without leakage.
These specialized specimen bag solutions directly tackle the persistent risks of breakage, leakage, and contamination during transit. The 95kPa-rated bags provide essential durability, particularly for high-altitude shipments where pressure changes pose a threat to sample integrity.
Crucially, manufacturers are aligning designs with stringent international regulations. Modern UN3373 sample bag systems incorporate mandatory markings and construction standards prescribed for transporting Category B biological substances (UN3373). This ensures seamless compliance across global logistics networks, reducing delays and regulatory penalties.
“The move towards integrated systems – combining certified UN3373 sample bags with robust 95kPa performance – represents a significant safety upgrade,” notes Dr. Lena Rossi, a clinical laboratory director. “It’s not just about the specimen carrier bag itself, but ensuring the entire chain – from clinic collection point to central lab – maintains containment.”
Field personnel, including phlebotomists and outbreak response teams, report increased confidence using these multi-layered specimen bag solutions. The combination of puncture resistance, validated pressure tolerance (95kPa), and clear UN3373 certification markings streamlines safe handling and reduces training complexity.
As global sample volumes surge, the evolution towards standardized, high-performance 95kPa bags meeting UN3373 sample bag requirements is setting a new benchmark for reliability in medical logistics.
Packaging:
Primary Containers: Must be leak-proof and puncture-resistant, holding only one patient’s specimen.
Secondary Containers: Should be sealable, leak-proof, and marked with a biohazard symbol.
Outer Containers: May be used for additional protection and temperature control, especially for long distances or sensitive samples.
Labeling: Clearly labeled with patient identification information, including medical record number, and other relevant details. Labels should be legible and unobstructed.
Secure Closures: All containers and packaging should be securely closed during transport.
Documentation: Accompanying paperwork should be protected from contamination and separate from the specimen.
Temperature Control: If required, specimens should be transported in a manner that maintains the appropriate temperature.
Handling: Specimens should be handled carefully to avoid contamination.
Regulations: Adherence to local, state, and federal regulations is essential.
Specialized Transport: Some specimens may require specialized transport, such as those containing infectious substances, which necessitates strict adherence to biohazard material transport regulations.
Couriers: Qualified couriers with knowledge of specimen handling, packaging, labeling, and relevant regulations are crucial.
Data Security: If digital systems are used, data security and patient confidentiality must be maintained.