How special are the packaging and labels of special items?
Release time: 2025-08-26
1.What are special items?
- Microorganisms: including viruses, bacteria, fungi, actinomycetes, rickettsia, spirochetes, chlamydia, mycoplasma and other medical microbial (toxic) species and samples as well as parasites and environmentally friendly microbial agents;
- Human tissues: including human cells, cell lines, embryos, organs, tissues, bone marrow, secretions, excretions, etc.;
- Biological products: including vaccines, antitoxins, diagnostic reagents, cytokines, enzymes and their preparations as well as toxins, antigens, antibodies, antigen-antibody complexes, allergens, nucleic acids, immunomodulators, microecological preparations and other biologically active preparations used in human medicine and life science related fields;
- Blood and its products: including human whole blood, plasma components, special blood components, and various human plasma protein products.
2.Specificity of packaging requirements
- The operating units using special items entering and leaving the country and the third-party packaging or transportation companies entrusted by them should ensure that the packaging and labeling of special items comply with the requirements of relevant laws and regulations.
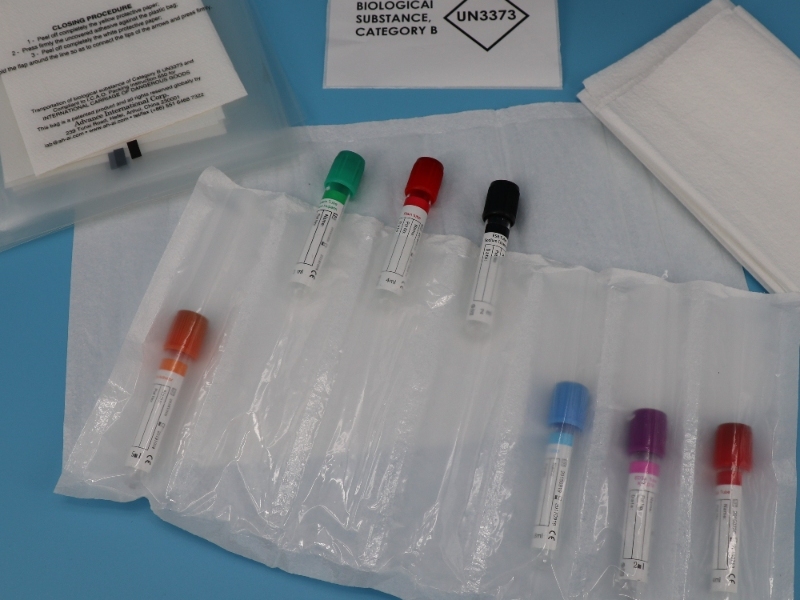
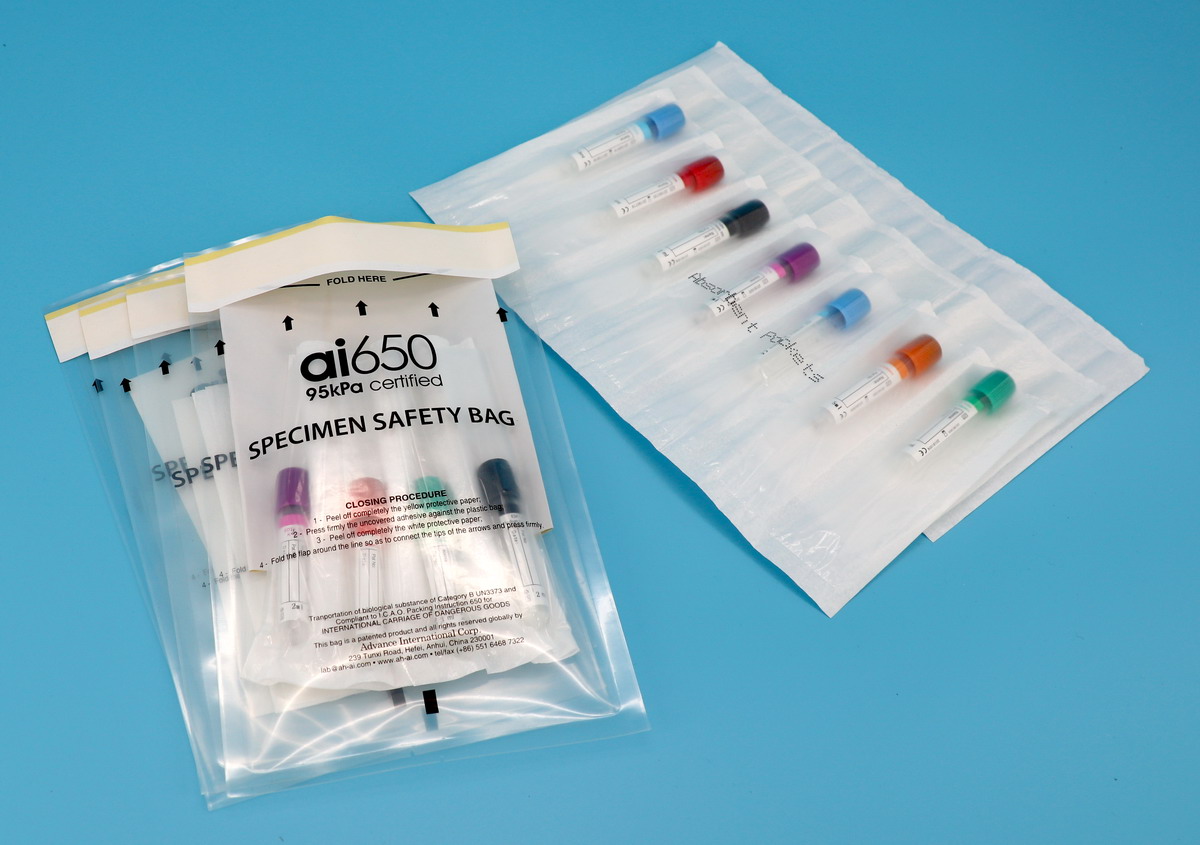
- The packaging of special items entering or leaving the country must be waterproof, leakproof and affixed with appropriate labels indicating the contents. The outer side of the inner packaging of high-risk special items must be wrapped with sufficient absorbent material so that all spilled liquids can be absorbed when the inner packaging is broken or leaked.
Note: Transport packaging classification: According to the classification and packaging requirements of ICAO document Doc9284 “Technical Instructions for the Safe Transport of Dangerous Goods by Air”, the relevant pathogens and standards are divided into two categories, A and B, and the corresponding UN numbers are UN2814 (animal viruses are UN2900) and UN3373. For Class A infectious substances, if the table does not indicate “dried virus cultures only”, all materials related to the virus are included; for Class A infectious substances indicated as “dry virus cultures only”, virus cultures are packaged according to UN2814, and other specimens are packaged according to UN3373 requirements. All viruses and related samples marked as Class B are packaged and transported by air according to the requirements of UN3373. For transportation by other means of transportation, the packaging can refer to the above standards. The packaging requirements for special items are too special and require too much professionalism. Please listen carefully. Let’s take a look at the special requirements for packaging labels
3.“Speciality” of label requirements
- The outer packaging label of special items should have the product name in Chinese or English. If there is a biosafety risk, it should have a biohazard label that meets the relevant requirements.
- The packaging label of special items imported for sale should have the product name, manufacturer name and address in Chinese. According to the characteristics and usage requirements of the product, if it is necessary to indicate the product specifications, grade, name and content of the main ingredients, it should be indicated in Chinese accordingly.
- The packaging of special items imported for scientific research purposes should have the name and specifications in Chinese or English, and indicate “For scientific research use only”.
- For non-commercial products imported abroad or for products whose packaging surface area is too small to be labeled, product materials containing the above information should be attached, but at least the product name should be included.