Mastering UN3373 Compliance: Essential Guide to Biological Sample Containers & Safe Transport
Release time: 2025-06-17
In the rapidly evolving fields of healthcare, diagnostics, and scientific research, the safe and compliant transportation of biological samples is not merely a logistical task—it is a foundational pillar for accurate diagnoses, successful clinical trials, and breakthrough discoveries. Biological samples are not inert commodities; they are complex matrices containing delicate biomolecules—such as DNA, RNA, proteins, and metabolites—that are highly susceptible to degradation from various environmental factors during transit. Compromised samples due to improper handling or non-compliant packaging can lead to invalidated research, misdiagnoses, and significant financial losses, underscoring the absolute necessity for precision and adherence to stringent regulations. Transporting samples on a global scale further amplifies these challenges, requiring specialized knowledge and expertise to navigate diverse climates and infrastructures. Every sample is time-sensitive and temperature-dependent, demanding specialized handling to maintain its integrity throughout the journey.
The United Nations (UN) has established a universal classification system for dangerous goods, with UN3373 specifically designating “Biological Substance, Category B”. This classification applies to human or animal materials that, while infectious, do not meet the criteria for Category A substances, which are defined by their capability to cause permanent disability, life-threatening, or fatal disease upon exposure. Adherence to UN3373 packaging and transport guidelines, primarily governed by the International Air Transport Association (IATA) Packing Instruction 650 (PI650), is non-negotiable for ensuring safety and regulatory compliance across domestic and international borders.
For entities involved in medical and scientific endeavors, consistently demonstrating rigorous compliance with these regulations is more than just avoiding penalties; it is a strategic investment. When an organization consistently adheres to stringent standards, such as those for UN3373 packaging, it actively builds a reputation for reliability, deep knowledge, and trustworthiness. This commitment to quality and safety becomes a powerful competitive advantage, attracting more clients and potentially commanding higher prices in the market. This is particularly true in areas related to health and safety, where accuracy and credibility are paramount for public well-being.
Furthermore, the concept of “safety” in medical logistics is continually expanding. While UN3373 substances are categorized as presenting a “relatively low risk” in the event of a release compared to Category A , the detailed packaging requirements and emphasis on “biosafety” underscore that even low risk demands high vigilance. The increasing prevalence of at-home testing means that the scope of individuals handling these packages extends beyond trained laboratory personnel to include postal workers and the general public. This broader exposure necessitates that manufacturers and shippers implement robust measures that consider a wider range of potential exposure points. A proactive approach to safety, anticipating and addressing these broader concerns, further enhances a company’s standing as a reliable and authoritative provider.
At Biospecimenbag.com, the understanding of the intricate demands of biological sample logistics is paramount. The commitment extends beyond merely supplying containers; the company provides rigorously tested, certified, and innovative packaging solutions designed to meet and exceed global UN3373 standards. This ensures the integrity of valuable specimens and the safety of all involved in the transport chain, aiming to be a steadfast partner, offering peace of mind through unparalleled expertise and reliable products.
II. Understanding UN3373: Decoding the Regulations for Category B Biological Substances
Biological Substance, Category B, identified by the UN number UN3373, refers to infectious substances that do not meet the criteria for Category A. These typically include diagnostic specimens, clinical specimens, and biological products that, while potentially infectious, are not capable of causing permanent disability, life-threatening, or fatal disease in otherwise healthy humans or animals upon exposure. Examples of such materials include blood and its components, tissue, tissue fluids, or body parts transported for purposes such as research, diagnosis, investigational activities, disease treatment, or prevention.
The safe transport of UN3373 biological samples is governed by a framework of international and national regulations. The International Air Transport Association (IATA) Dangerous Goods Regulations (DGR) are central, with Packing Instruction 650 (PI650) serving as the cornerstone for air transport of UN3373 substances. PI650 outlines detailed requirements for packaging, labeling, and documentation, making compliance mandatory for air shipments. For domestic shipments within the United States, the Department of Transportation (DOT)’s Hazardous Materials Regulations (HMR) align closely with international standards, ensuring consistent safety protocols. The World Health Organization (WHO) also provides guidance and recommendations that often form the basis for national and international regulations, emphasizing public health safety.
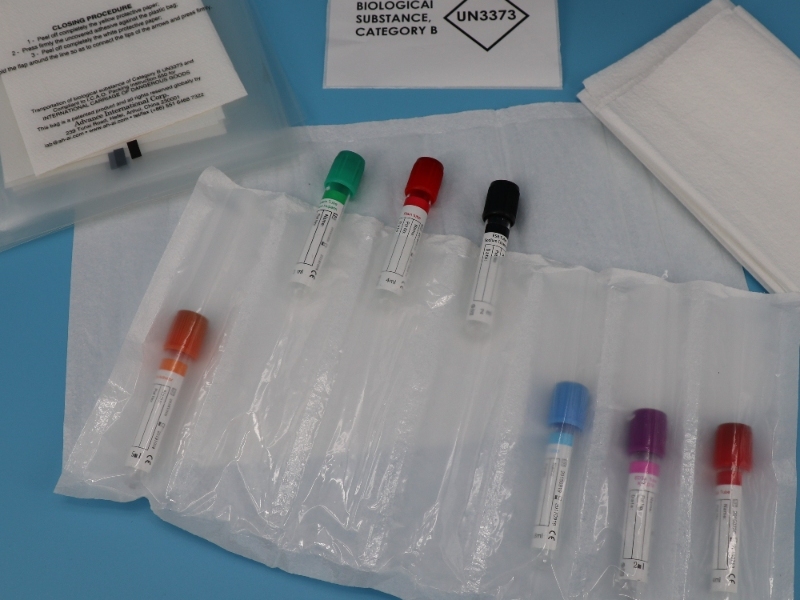
The Triple Packaging System: A Cornerstone of UN3373 Compliance
The triple packaging system is the mandatory standard for all Category B infectious substances, meticulously designed to contain the sample securely even if one layer fails. This robust system comprises three distinct components:
- Leak-proof Primary Receptacle: This innermost container directly holds the biological sample. For liquids, it must be leak-proof and securely sealed, often with a screw-on cap reinforced with tape or parafilm. For solids, it must be sift-proof. The maximum volume for a primary receptacle is 1 Liter for liquids, and for solids, it must not exceed the outer packaging weight limit. Primary receptacles can be made of glass, metal, or plastic, provided they offer a positive, leak-proof seal.
- Leak-proof Secondary Packaging: This intermediate container encloses and protects the primary receptacle(s). It must be leak-proof for liquids and sift-proof for solids. A critical requirement for air transport is that either the primary or secondary packaging must be capable of withstanding an internal pressure differential of not less than 95 kPa (0.95 bar) across a temperature range of -40°C to +55°C (-40°F to 130°F) without leakage. This pressure resistance is crucial because biological samples transported by air are subject to significant air pressure changes at high altitudes, which could otherwise lead to specimen leakage or even the bursting of the container, posing risks to handling personnel. If multiple fragile primary receptacles are placed within a single secondary packaging, they must be individually wrapped or separated to prevent contact and breakage. Sufficient absorbent material (e.g., cellulose wadding, cotton balls, super-absorbent packets, paper towels) must be placed between the primary and secondary packaging to absorb the entire contents of all primary receptacles in case of leakage, preventing compromise to the cushioning or outer packaging.
- Rigid Outer Packaging: This outermost layer provides physical protection to the entire system during transport. It must be rigid, made of materials like corrugated fiberboard, wood, metal, or rigid plastic, and strong enough to withstand shocks, loadings, and a 1.2-meter (4-foot) drop test without leakage. The smallest external dimension of the outer packaging must be at least 100x100mm. Cushioning material, distinct from absorbent material, should be placed between the secondary and outer packaging to prevent excessive movement and protect fragile contents. The maximum quantity per outer container is 4 Liters for liquids or 4 kg for solids, excluding coolants like ice or dry ice.
The consistent emphasis on the 95kPa pressure resistance across regulations highlights its role as a fundamental requirement for air transport of Category B biological samples. Manufacturers and suppliers who meet this stringent standard are positioned to serve a much broader market, including international logistics, thereby enhancing their standing and reliability in the industry. The ability of packaging to maintain integrity across a wide temperature range during this pressure test further demonstrates robust engineering and a commitment to safety under diverse conditions.
The strength of the triple packaging system lies not merely in its individual components, but in their synergistic interaction. The design ensures that a failure in one layer, such as a leak from the primary receptacle, is effectively mitigated by the subsequent layers. The absorbent material within the secondary packaging contains any spills, preventing them from compromising the cushioning or the outer packaging. This layered defense ensures comprehensive safety and preserves sample integrity, demonstrating that the system’s resilience is a result of its integrated design and rigorous testing of the entire protective chain.
UN3373 Packaging Requirements: Liquid vs. Solid Substances
| Feature / Requirement | For Liquid Substances (e.g., Blood, Urine) | For Solid Substances (e.g., Tissue, Dried Blood Spots) |
| Primary Receptacle | Leak-proof; Max 1L per receptacle | Sift-proof; Must not exceed outer packaging weight limit |
| Secondary Packaging | Leak-proof; Withstand 95kPa pressure (-40°C to 55°C | Sift-proof; Withstand 95kPa pressure (-40°C to 55°C) |
| Absorbent Material | Required, sufficient to absorb entire contents of primary receptacle(s) | Required if residual liquid may be present; otherwise, not explicitly required |
| Outer Packaging | Rigid; Max 4L per outer container (excluding coolants); Min 100x100mm external dimension; 1.2m drop test | Rigid; Max 4kg per outer container (excluding coolants); Min 100x100mm external dimension; 1.2m drop test |
| Multiple Primary Receptacles | Individually wrapped or separated to prevent contact within secondary packaging | Individually wrapped or separated to prevent contact within secondary packaging |
Mandatory Testing Standards: To ensure compliance, packaging components must undergo rigorous testing:
- Pressure Test (95kPa): As noted, the primary or secondary receptacle for liquids must withstand an internal pressure differential of 95 kPa, and for air transport, this must be maintained within a temperature range of -40°C to +55°C. This test is vital to prevent leakage or bursting due to changes in air pressure during transit.
- Drop Test: The completed outer package must be capable of withstanding a 1.2-meter (4-foot) drop test without leakage from the primary receptacle. This simulates potential impacts during handling and transport, ensuring the packaging is robust enough to withstand typical mishaps during its journey.
- Puncture Test: While less frequently detailed specifically for Category B, the general principle for dangerous goods packaging includes puncture resistance to ensure primary receptacles remain intact even if the outer packaging is compromised. A puncture test involves subjecting packages to a cylindrical steel rod drop, with the aim that primary receptacles contain any leakages, even if the secondary packaging is affected.
Labeling and Documentation: Ensuring Visibility and Accountability
The outer packaging of a UN3373 shipment must clearly display specific markings to ensure proper identification and handling. These include the UN3373 diamond-shaped mark (a square set at a 45-degree angle, with each side at least 50mm long, and line width at least 2mm). The Proper Shipping Name, “BIOLOGICAL SUBSTANCE, CATEGORY B,” must be prominently displayed adjacent to the diamond mark, with letters and numbers at least 6mm high. Additionally, the sender’s name and address, and the recipient’s name and address are required. A critical safety measure is the inclusion of the name and telephone number of a responsible person, available 24/7 for emergencies during transport, which can be on the package or the air waybill. For liquid shipments, orientation arrows are required, affixed on two opposite sides and perpendicular to the front of the package, in red or black on a contrasting background. If an overpack (an extra outside packaging for multiple boxes or refrigeration) is used, all markings and labels from the inner package must be visible or replicated on the overpack, along with the word “OVERPACK”.
The requirement for a 24-hour emergency contact, the “responsible person,” on the package or air waybill is more than an administrative detail; it represents a critical human element in the safety chain. In the event of an incident such as leakage or spillage, immediate expert guidance is necessary. This regulatory mandate implicitly demands robust internal training programs within organizations that ship biological samples, ensuring that personnel are not just compliant but truly competent in emergency response. This deepens the reliability and expertise of any company operating in this sector.
While Category B shipments are less stringent than Category A, specific documentation is still mandatory. An itemized list of contents must be enclosed between the secondary and outer packaging. For air transport, the Air Waybill must include the text “Biological Substance, Category B” and “UN3373,” along with the number of packages, and the “Dangerous Goods ‘YES'” box should be ticked. It is important to note that, unlike Category A substances, UN3373 Category B shipments generally do not require a Shipper’s Declaration for Dangerous Goods (DGD). This simplifies the documentation process but does not diminish the importance of other required markings and lists.
A potential area of confusion for shippers lies in the distinction between “exempt human/animal specimens” and fully regulated UN3373 substances. While certain specimens with a minimal likelihood of containing pathogens may be exempt from full UN3373 regulations, they still require leak-proof triple packaging and specific marking, such as “Exempt human specimen” or “Exempt animal specimen”. This highlights that even for seemingly “exempt” materials, robust packaging practices are often necessary. This situation underscores the need for clear, accessible guidance from packaging providers to help customers correctly classify their samples and choose appropriate packaging, thereby preventing accidental non-compliance and enhancing the provider’s expertise and reliability.
III. Navigating the Complexities: Common Challenges in Biological Sample Logistics
The inherent fragility of biological samples presents significant challenges that extend beyond mere regulatory compliance. These challenges, if not adequately addressed, can compromise sample integrity and undermine the validity of critical research and diagnostic efforts.
Maintaining Sample Integrity:
- Temperature Fluctuations: Many biological samples, including blood, tissue, and vaccines, are highly sensitive to temperature changes. Deviations from optimal temperature ranges—such as 15-30°C for ambient, 2-10°C for refrigerated, or -20°C to -80°C for frozen—can lead to severe consequences. High temperatures can cause denaturation of biomolecules like proteins and enzymes, rendering them biologically inactive. Conversely, unstable cold temperatures can compromise biomarker stability and denature proteins, leading to spoilage. Improper temperature control can also promote microbial growth and contamination. This necessitates robust cold chain management throughout the entire transport process.
- Physical Shock and Degradation: Samples are vulnerable to physical impacts, vibration, and rough handling during transit. Such mechanical stressors can cause breakage of primary receptacles, leading to leakage, mixing of contents, or cellular damage that compromises the sample’s viability. Adequate cushioning material, distinct from absorbent material, is therefore essential to protect fragile contents and limit movement within the packaging. Robust outer packaging is crucial to absorb external shocks and protect the inner components.
- Prolonged Transit Times: Extended shipping durations significantly increase the risk of temperature excursions and degradation, especially for time-sensitive specimens. The longer a sample is in transit, the greater the likelihood it will encounter conditions outside its optimal range, potentially leading to spoilage and rendering it useless for analysis.
The various physical stressors encountered during transport, such as temperature fluctuations, physical shock, and prolonged transit times, are not isolated threats; they often interact to accelerate sample degradation. For instance, a primary container that experiences a slight compromise due to physical shock might become more susceptible to leakage if subsequently subjected to pressure changes (underscoring the importance of 95kPa resistance) or further temperature fluctuations. Similarly, extended transit periods exacerbate both temperature and physical stress risks. This highlights the need for packaging solutions that offer holistic protection against a combination of environmental challenges, rather than merely addressing individual ones. This comprehensive approach reinforces the value of rigorously tested, integrated triple packaging systems.
Regulatory Patchwork: Navigating the diverse and often overlapping regulatory requirements across different jurisdictions (e.g., IATA for air, ADR for road, national DOT regulations) can be complex and challenging. Misunderstandings or insufficient documentation can lead to costly customs delays and non-compliance, particularly in international shipping. Staying informed about varying regulations and ensuring all necessary permits and licenses are obtained before shipment is critical for smooth logistics.
Risk Mitigation: Preventing Leaks, Contamination, and Security Breaches:
- Leakage and Contamination: The primary risk associated with UN3373 substances is the potential release of potentially infectious materials. Even though Category B substances present a “relatively low risk” compared to Category A, any release poses hazards to personnel, including laboratory staff, couriers, and postal workers, as well as to the environment. This is precisely why leak-proof primary and secondary components, coupled with sufficient absorbent materials, are paramount to contain any spills and prevent contamination.
- Security and Tampering: Ensuring that samples are not tampered with or accessed by unauthorized individuals is vital for maintaining the chain of custody and data integrity, especially for forensic or high-value research samples. Technologies like tamper-evident seals and holographic labels are increasingly used to protect products from unauthorized access and prevent counterfeiting.
While technological advancements in packaging are crucial, the human element remains a critical factor in the safety and integrity of biological sample transport. Challenges such as complacency, incorrect tube filling, improper use of personal protective equipment (PPE), and a general lack of training among personnel can undermine even the most advanced packaging solutions. This emphasizes that packaging providers have a significant role beyond supplying products; they must also contribute to the education and training of their clients. By providing clear, user-friendly instructions and educational resources, companies can empower their customers to correctly handle and prepare shipments, thereby mitigating a significant, often overlooked, risk factor and reinforcing the provider’s overall reliability and expertise.