Understanding UN3373 Biological Specimen 95kPa Biohazard Bags
Release time: 2025-07-02
In the world of medical and biological research, the safe transportation of diagnostic specimens is paramount. These specimens, which can include blood, tissues, and other bodily fluids, are crucial for diagnosis, research, and public health monitoring. However, transporting such materials poses significant risks if not done properly. This is where UN3373 Biological Substance, Category B, and the 95kPa Biohazard Bag come into play.
What is UN3373?
UN3373 is the United Nations number assigned to Biological Substance, Category B. This category includes diagnostic specimens that are being transported for the purpose of diagnosis or investigation. These specimens are not believed to contain pathogens that are likely to cause permanent disability or life-threatening diseases in humans or animals. Examples include:
- Blood and its components
- Excreta
- Tissues and other bodily fluids
Unlike Category A substances, which are known or suspected to contain pathogens that can cause severe disease, Category B substances are considered lower risk but still require adherence to specific transport regulations. Certain substances are exempt from UN3373 regulations, such as:
- Non-infectious substances or those unlikely to cause disease
- Non-pathogenic microorganisms
- Neutralized or inactivated pathogens
- Drained medical equipment
- Low-risk environmental samples (e.g., food, water)
- Dried blood spots, faecal occult blood tests, or samples for transfusion/transplantation
- Specimens with minimal pathogen likelihood, marked as “Exempt human specimen” or “Exempt animal specimen”
Even exempt specimens must follow specific packaging guidelines to ensure safety during transport.
The Role of 95kPa Biohazard Bags
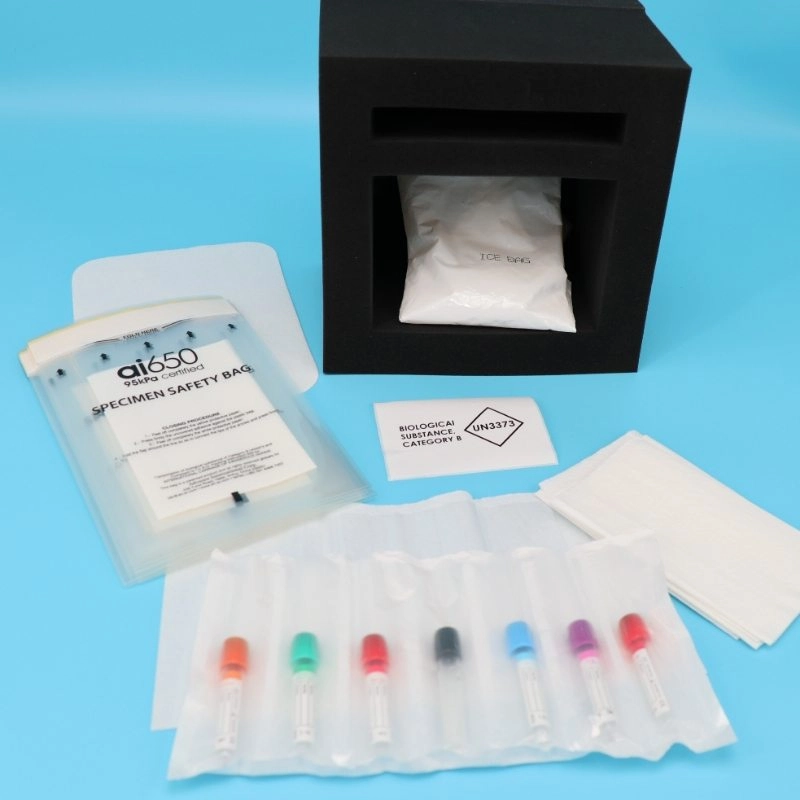
To ensure the safe transport of these biological substances, especially by air, the use of 95kPa Biohazard Bags is essential. These bags are designed to withstand a pressure differential of 95kPa, which is the standard required for air transport to prevent leakage due to changes in atmospheric pressure during flight. The 95kPa specification ensures that the packaging maintains its integrity under varying pressures, protecting both the sample’s integrity and the safety of those handling the package. These bags comply with international regulations, including those set by the International Air Transport Association (IATA), the International Civil Aviation Organization (ICAO), and the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR).
Features of 95kPa Biohazard Bags
95kPa Biohazard Bags are engineered with several key features to meet the rigorous standards for transporting biological substances:
Leak-Proof and Puncture-Resistant: Made from high-quality materials that prevent leaks and punctures, ensuring that the contents remain contained.
Absorbent Materials: Include absorbent pads or layers to soak up any liquids, minimizing the risk of leakage.
Secure Closure: Equipped with strong adhesive strips or other secure closure mechanisms to keep the bag sealed during transport.
Labeling and Documentation: Often come with spaces for necessary labels and documentation, such as the UN3373 mark, to indicate the contents and handling instructions.
Many shipping companies, such as FedEx, offer pre-approved packaging solutions like the FedEx UN 3373 Pak, which includes 95kPa Biohazard Bags or equivalent, ensuring compliance with all regulatory requirements. Using certified packaging is critical to avoid compliance issues.
| Feature | Description |
| Leak-Proof Design | Prevents leakage of liquids, ensuring safe containment of biological specimens. |
| Puncture Resistance | Durable materials protect against tears or punctures during transport. |
| Absorbent Layer | Soaks up any liquid spills, reducing risk of exposure. |
| Secure Closure | Strong adhesive or sealing mechanisms keep the bag securely closed |
| Regulatory Compliance | Meets IATA, ICAO, and ADR standards for safe transport of UN3373 substances. |
Applications and Usage
95kPa Biohazard Bags are widely used in various settings, including:
Hospitals and Clinics: For transporting patient samples to laboratories for testing.
Research Laboratories: For shipping samples between facilities for collaborative research.
Pharmaceutical Companies: For clinical trials and quality control testing.
Public Health Agencies: For disease surveillance and outbreak response.
Proper packaging is critical for compliance and safety. The process typically involves:
- Primary Receptacle: Place the specimen in a leak-proof container (e.g., glass, metal, or plastic with a secure seal).
- Secondary Packaging: Enclose the primary container in a 95kPa Biohazard Bag, ensuring it is sealed properly.
- Outer Packaging: Place the secondary packaging in a sturdy outer box (e.g., corrugated fiberboard) with at least one surface measuring 100 mm x 100 mm, capable of withstanding a 4-foot drop test.
- Absorbent Material: Include absorbent materials (e.g., cellulose wadding, cotton balls) for liquid samples to absorb the entire contents in case of a leak.
- Labeling: Affix the UN3373 diamond mark (minimum 50 mm per side) with the text “Biological Substance, Category B” in 6-mm-high letters, along with shipper and recipient details and a responsible person’s contact information.
Ensuring Compliance and Safety
Transporting biological substances is heavily regulated to protect public health and the environment. Compliance with IATA, ICAO, and ADR regulations is mandatory, specifying requirements for packaging, labeling, and documentation. For example:
Volume/Weight Limits: Liquid samples are limited to 1 L per primary receptacle and 4 L per outer container; dried samples are limited to 4 kg per outer container.
Marking Requirements: The UN3373 mark must be displayed on the outer packaging, along with proper shipping names and contact details.
Packaging Standards: The outer packaging must be rigid and sturdy, and chipboard or paperboard is not acceptable.
Failure to comply can result in legal penalties, shipping delays, and risks to public health. Using certified 95kPa Biohazard Bags and adhering to packaging guidelines is essential for safe and compliant transport.

![[95kpa sterile sample bags] 2025-2031 Global and Chinese sterile sample bags market status and future development trends](https://lingjuimg.com/wp-content/uploads/AIC/2025/08/transport-bag-220.webp)