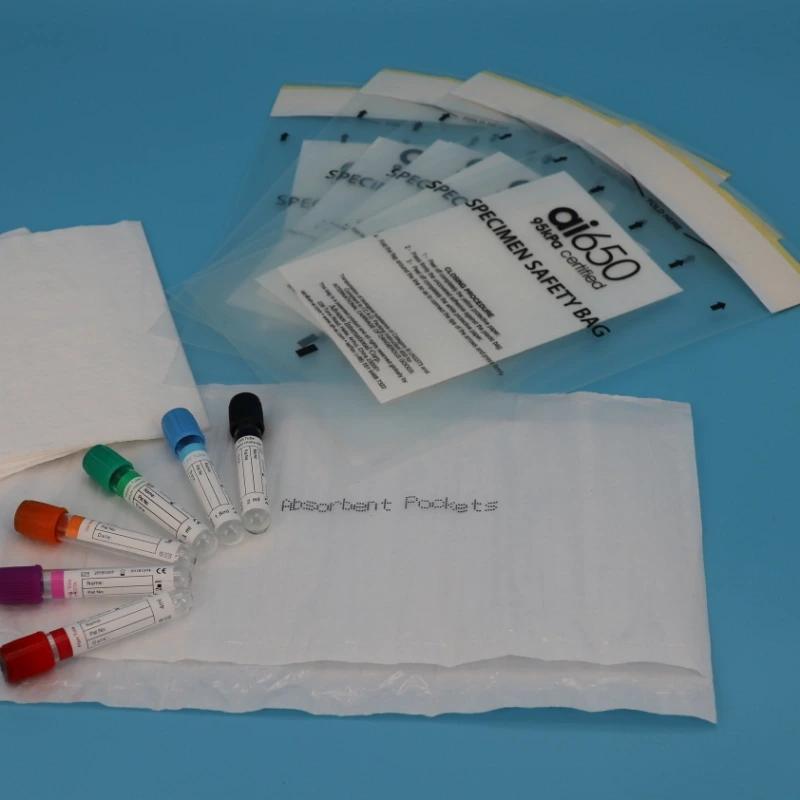
Why Should Biological Specimen Bags use Certified 95 kPa Pressure Bags?
Release time: 2025-08-06
In medical laboratories, blood samples containing highly pathogenic pathogens often require biological hazard specimen bags for transportation. The use of certified bags directly relates to the biological safety of the entire transportation chain. A bag with a clear “95kPa” and biological hazard symbol printed on it represents a key safety barrier that has passed strict certification. In the global field of biological sample transportation, these certified 95kPa pressure bags are no longer just ordinary packaging materials; they are mandatory equipment to protect public health safety.
Core Requirements of International Standards
The International Air Transport Association (IATA) and the United Nations’ “Recommendations on the Transport of Dangerous Goods” explicitly stipulate that the transport of UN3373 (Class B biological substances) must use secondary packaging bags tested to withstand 95kPa pressure. This technical specification requires the packaging to endure pressure changes equivalent to the difference in pressure between 5000 meters above sea level and the ground, ensuring the container does not leak at extreme temperatures ranging from -40°C to +55°C.
China’s “Regulations on the Safety Management of Pathogenic Microorganisms in Laboratories” also stipulates that the transportation of high-pathogenic microorganisms and samples must use packaging certified by nationally designated laboratories. The core requirement is that the packaging must be capable of withstanding 95kPa pressure. Ordinary plastic bags without certification are prone to expansion and rupture in low-pressure environments, which can lead to the leakage of infectious substances and cause catastrophic contamination.
Technical Features of 95kPa Certified Sample Bags
Multi-layer Protection Design
- High-strength composite material: Made of special polyethylene or polyester materials with a thickness and toughness treated to maintain high transparency and tear resistance. Even if the internal container ruptures unexpectedly, the bag can effectively prevent the leakage of liquids and bioaerosols.
- Patented sealing system: Through a special adhesive and folding seal design (such as the “Tear-Fold-Adhere” three-step sealing of AI650), the seal will not open under pressure changes. The adhesive layer does not become brittle at -40°C and does not soften at +55°C.
- Tamper-evident marking: Once the seal is broken, clear marks are left, preventing unauthorized opening during transportation.
Integrated Functional Accessories
- Biohazard symbols: Prominent “Biohazard” and UN3373 labels in accordance with international hazardous goods marking regulations.
- Document isolation pouch: Transparent external pouch to store shipping documents, reducing the risk of breakage during opening.
- Absorbent pad slots: Some models come with multi-slot absorbent bags (such as the AI650 seven-slot absorbent pad), which can absorb up to 550ml of liquid and quickly lock in contaminants if leakage occurs.
Key Application Scenarios and Operational Standards
High-Risk Biological Sample Transportation
- Pathogen transportation: Such as COVID-19 virus strains, HIV blood samples, and tuberculosis cultures (UN2814/UN2900), which must use a “three-layer packaging system” — primary container + 95kPa sample bag + rigid outer box.
- Clinical diagnostic samples: Pathological samples (such as tumor tissue slices) transferred between hospitals and epidemiological investigation samples (UN3373) sent to disease control centers.
- Vaccine research and logistics: Biological active substances, such as attenuated strains and recombinant vectors, that require cold chain transportation.
Safety Assurance Under Harsh Conditions
A real-world example from an international laboratory in 2022 is telling: a batch of Ebola virus testing blood samples was transported by air from an African outbreak zone to a European reference laboratory. During the transport, the samples endured 45°C high-temperature layovers and -18°C conditions in the cargo hold. The 95kPa sample bags, despite expanding nearly double their size due to pressure changes, successfully contained the leak caused by a cracked test tube, thanks to their certified materials.
Critical Operational Requirements
Mandatory Accompanying Measures
- Use of absorbent materials is compulsory: When transporting liquid samples, absorbent pads must be placed inside the bag, such as AI650’s seven-slot absorbent pad.
- Rigid outer packaging protection: The sample bag must be placed inside a foam box and waterproof outer box that meet puncture resistance standards.
- Temperature-sensitive sample handling: When using cooling agents, avoid direct pressure on the bag with ice packs to prevent low-temperature brittleness.
Prohibited Procedures and Guidelines
- No reuse: This product is a single-use consumable. Reusing it requires professional disinfection, but biological risks remain.
- Sealing procedure standard: The AI650 bag must follow the “three-step sealing method”: ①Place sample into the inner bag → ②Tear off the seal protection film → ③Fold and adhere to seal the opening.
- Environmental precautions: Store away from ultraviolet rays (sunlight can age materials) and corrosive chemicals.
Choosing and Verifying Certified Products
To distinguish compliant products, look for three key markings:
- Testing report number: Indicating testing by an ISO17025 certified laboratory.
- Standard code: Indicating compliance with IATA PI650/PI602 or ADR P650 standards.
- Temperature tolerance range: Clearly marking “-40°C to +55°C, 95kPa pressure resistance” performance parameters.
Biological Safety Warning: The case of Advance International Corp. highlights industry risks — some organizations use ordinary sealed bags to impersonate 95kPa bags in order to save costs. In 2023, a batch of malaria samples leaked during air transport due to the use of uncertified packaging, resulting in the suspension of international supply qualifications.
As global collaboration in the biomedicine sector deepens, from hospital labs to WHO international projects, 95kPa biological sample bags have become the “mobile ark” guarding biological safety. Ensuring that every sealing action follows the standard not only complies with operational procedures but also demonstrates respect for life.