Why the 95 kPa Pressure Bag is a Key Choice for Safe Specimen Transport
Release time: 2025-07-10
In the transportation of precious tumor samples traversing clinical research organizations, infectious pathogens urgently needed by medical service centers, and genetic materials being transferred across borders in biological laboratories—these high-value, high-risk biological specimens’ transport safety is critical to research progress and patient health. A small packaging mistake during transport could result in sample failure, data loss, or even biological safety risks. Choosing the right transport packaging is never just a simple “packing” task but the first line of defense in safeguarding scientific rigor and public safety.
95 kPa: The Scientific Threshold for Specimen Safety
For packaging biological hazardous materials such as UN 2814 (infectious substances affecting humans), UN 2900 (infectious substances affecting animals), and UN 3373 (Class B diagnostic specimens/clinical waste), the International Air Transport Association (IATA) and national regulations (such as China’s “Regulations on the Air Transport of Dangerous Goods”) have strict guidelines. Among these, the 95 kPa pressure test simulating the low-pressure environment of aviation is the core indicator to verify packaging integrity.
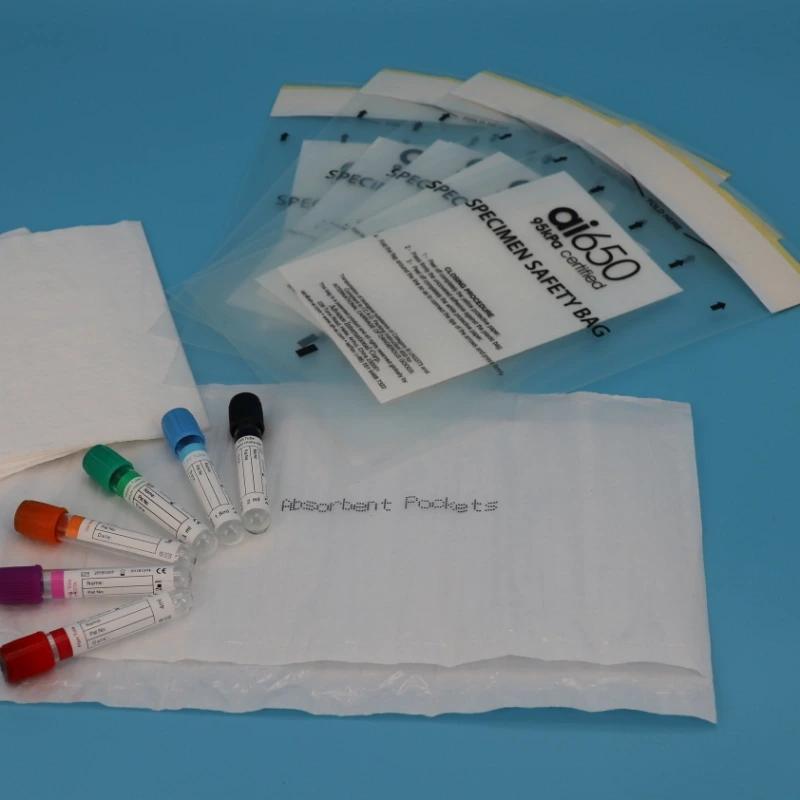
The AIC biological sample transport bag is specifically designed to meet this crucial need:
- Precise Pressure Protection: Optimized to withstand the 95 kPa low-pressure environment, ensuring that the specimens do not rupture or leak in the high-altitude cargo hold, keeping the specimens safely isolated from the environment.
- Triple Certification Protection: Strictly complies with the packaging standards of UN 2814, UN 2900, and UN 3373, providing regulatory-compliant protection.
- Reliable Seal Barrier: Made with high-strength, puncture-resistant composite materials and precise sealing technology, effectively resisting transport vibrations and unexpected impacts.
Industry Pain Points Reflect the Value of the 95 kPa Pressure Bag
- Clinical Research and Trial Organizations: In multinational multi-center trials, if valuable patient tissue samples are damaged or contaminated during transport, the entire research schedule could be delayed by months. Using a certified 95 kPa pressure bag, along with a compliant three-layer packaging system (primary container with absorbent material, AIC transport bag, and sturdy outer box), forms the foundation to ensure sample traceability and data integrity.
- Hospital Laboratories and Independent Laboratories: Daily transportation of samples for tests like Hepatitis B, HIV, etc. (UN 3373) also carries significant risk. Using regular plastic bags or inferior transport bags can cause them to burst under low-pressure conditions. A dedicated 95 kPa pressure bag, along with proper labeling, can significantly reduce the risk of leakage, ensuring the safety of logistics personnel and avoiding costly contamination cleanup and regulatory penalties.
- Biological Sample Banks and Pharmaceutical Research & Development: Biological sample banks store tens of thousands of genetic disease samples, which are the cornerstone of research. Reliable transport bags and temperature control during transport are just as important when shipping these long-stored samples to partner institutions. The 95 kPa pressure bag ensures the physical safety of the samples during pressure changes, maintaining the reputation of the biological bank and the value of the samples.
Selection and Usage Recommendations: Safety Cannot Be Compromised
- Strict Verification of Compliance: Always confirm that the transport bags are clearly labeled as compliant with UN 3373, UN 2814, or UN 2900 (depending on the contents) and meet the 95 kPa pressure test standards, with the corresponding testing report.
- Three-Layer Packaging is Essential: The AIC transport bag serves as the “secondary leak-proof container” (middle layer). The inner layer should be a sealed, leak-proof primary container (e.g., sterile sealed sample tubes), and the outer layer should be a sturdy corrugated cardboard box filled with cushioning materials.
- Proper Labeling and Documentation: Clearly label the biological hazard symbol, UN number, sender/recipient information, and attach necessary transport documents (such as a Dangerous Goods Declaration).
- Adapt to Sample Volume: Select the appropriate capacity transport bag based on the sample volume and primary container size to avoid overfilling or insufficient space.
- Regular Inspection and Replacement: Inspect the bags before use to ensure they are intact, with effective seals. Do not reuse single-use biological safety transport bags.
Safety Is Non-Negotiable, Compliance Is the Bottom Line
In the chain of biological sample transport safety, a transport bag that complies with the 95 kPa standard is a silent guardian protecting life codes, research breakthroughs, and public health. AIC will continue to deepen its efforts in the field of biological safety transport and provide industry-compliant and reliable packaging solutions. When you package precious samples next time, make sure to confirm: Does your transport bag truly cross the 95 kPa safety threshold?
The AIC technical team is available for packaging compliance consultation and verification support to ensure your biological sample transport is worry-free.